Palareti G, Cosmi B, Legnani C, et al.; DULCIS Investigators. D-dimer to guide the duration of anticoagulation in patients with venous thromboembolism: a management study. Blood. 2014 Jul 10;124(2):196-203.
Full-text for Emory users.
The optimal duration of anticoagulation in patients with venous thromboembolism (VTE) is uncertain. We investigated whether persistently negative D-dimers in patients with vein recanalization or stable thrombotic burden can identify subjects at low recurrence risk. Outpatients with a first VTE (unprovoked or associated with weak risk factors) were eligible after at least 3 months (12 in those with residual thrombosis) of anticoagulation. They received serial D-dimer measurements using commercial assays with predefined age/sex-specific cutoffs and were followed for up to 2 years. Of 1010 patients, anticoagulation was stopped in 528 (52.3%) with persistently negative D-dimer who subsequently experienced 25 recurrences (3.0% pt-y; 95% confidence interval [CI], 2.0-4.4%). Of the remaining 482 patients, 373 resumed anticoagulation and 109 refused it. Recurrent VTE developed in 15 patients (8.8% pt-y; 95% CI, 5.0-14.1) of the latter group and in 4 of the former (0.7% pt-y; 95% CI, 0.2-1.7; hazard ratio = 2.92; 95% CI, 1.87-9.72; P = .0006). Major bleeding occurred in 14 patients (2.3% pt-y; 95% CI, 1.3-3.9) who resumed anticoagulation. Serial D-dimer measurement is suitable in clinical practice for the identification of VTE patients in whom anticoagulation can be safely discontinued. This study was registered at clinicaltrials.gov as #NCT00954395.
Tritschler T, et al. Venous Thromboembolism: Advances in Diagnosis and Treatment. JAMA. 2018 Oct 16;320(15):1583-1594. Erratum in: JAMA. 2018 Dec 18;320(23):2486.
“D-dimer is a sensitive marker for VTE and excludes VTE without need for further testing among patients with a low clinical probability of PE.17-20 D-dimer levels greater than 500 ng/mL suggest the presence of PE. However, as D-dimer increases with age, older patients more often have false-positive test results, which lowers the test’s specificity in these patients. The false-positive rate can be reduced by using an age-adjusted D-dimer threshold, calculated as the patient’s age multiplied by 10 ng/mL (fibrinogen-equivalent units) for patients older than 50 years. When tested, the proportion of patients in whom imaging could safely be withheld based on a “PE-unlikely” Wells score and age-adjusted normal D-dimer levels increased from 28% to 33%.16 Age-adjusted D-dimer testing is useful when PE is suspected, although this approach appears to be less successful for inpatients and patients with previous VTE or cancer.16 Prospective validation of the age-adjusted D-dimer threshold to rule out DVT is currently ongoing (ClinicalTrials.gov identifier: NCT02384135).”
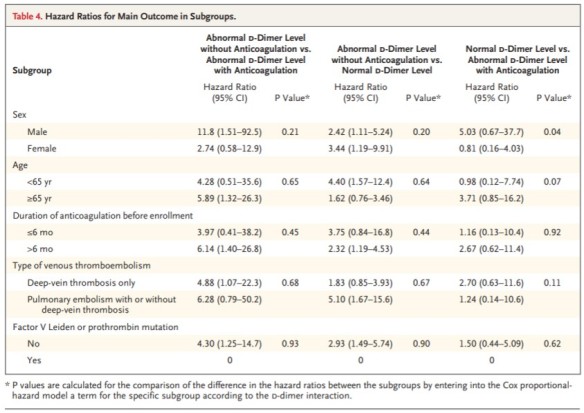
Palareti G, et al.; PROLONG Investigators. D-dimer testing to determine the duration of anticoagulation therapy. N Engl J Med. 2006 Oct 26;355(17):1780-9. Erratum in: N Engl J Med. 2006 Dec 28;355(26):2797.
Full-text for Emory users.
Results: The D-dimer assay was abnormal in 223 of 608 patients (36.7%). A total of 18 events occurred among the 120 patients who stopped anticoagulation (15.0%), as compared with 3 events among the 103 patients who resumed anticoagulation (2.9%), for an adjusted hazard ratio of 4.26 (95% confidence interval [CI], 1.23 to 14.6; P=0.02). Thromboembolism recurred in 24 of 385 patients with a normal D-dimer level (6.2%). Among patients who stopped anticoagulation, the adjusted hazard ratio for recurrent thromboembolism among those with an abnormal D-dimer level, as compared with those with a normal D-dimer level, was 2.27 (95% CI, 1.15 to 4.46; P=0.02).
Conclusions: Patients with an abnormal D-dimer level 1 month after the discontinuation of anticoagulation have a significant incidence of recurrent venous thromboembolism, which is reduced by the resumption of anticoagulation. The optimal course of anticoagulation in patients with a normal D-dimer level has not been clearly established. (ClinicalTrials.gov number, NCT00264277 [ClinicalTrials.gov].).
More PubMed results on D-dimer protocol for VTEs.