“Gastrointestinal stromal tumor (GIST) is the most common sarcoma and also a paradigmatic model for precision medicine in solid tumors, with the tyrosine kinase inhibitor
imatinib as a standard first-line treatment in the advanced phase and as adjuvant treatment in KIT- or PDGFRA-mutated GIST.”
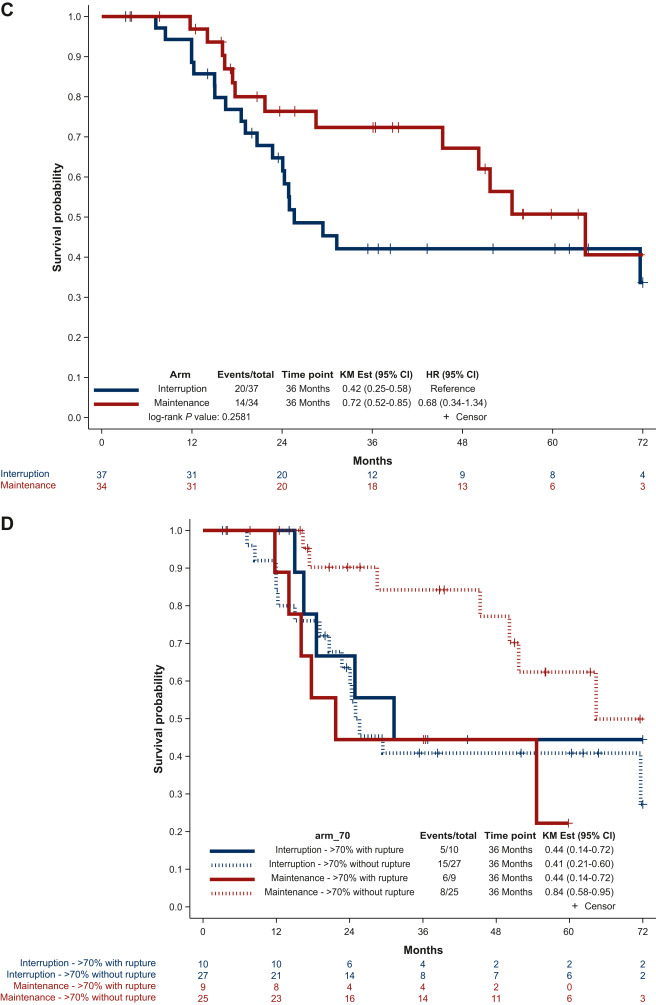
Whether a longer duration of imatinib treatment improves disease-free survival (DFS) has not been explored in a randomized setting. The randomized IMADGIST study was initiated in 2014 with the aim to determine whether the prolongation of adjuvant imatinib during 3 additional years improves the outcome of high-risk GIST patients versus a standard total duration of 3 years as recommended by clinical practice guidelines. The primary endpoint was DFS. We report here the results of this clinical study.”