One discussion this week included total neoadjuvant therapy (TNT) for rectal cancer.
Reference: Cercek A, et al. Adoption of total neoadjuvant therapy for locally advanced rectal cancer. JAMA Oncology. 2018 Jun 14;4(6):e180071. doi:10.1001/jamaoncol.2018.0071.
Summary: Treatment of locally advanced rectal (LARC) cancer involves chemoradiation, surgery, and chemotherapy. The concept of total neoadjuvant therapy (TNT), in which chemoradiation and chemotherapy are administered prior to surgery, has been developed to optimize delivery of effective systemic therapy aimed at micrometastases.
OBJECTIVE: To compare the traditional approach of preoperative chemoradiation (chemoRT) followed by postoperative adjuvantchemotherapy with the more recent TNT approach for LARC.
METHODS: A retrospective cohort analysis using Memorial Sloan Kettering Cancer Center (MSK) records from 2009 to 2015 was carried out. A total of 811 patients who presented with LARC (T3/4 or node-positive) were identified; 320 received chemoRT with planned adjuvant chemotherapy and 308 received TNT (induction fluorouracil- and oxaliplatin-based chemotherapy followed by chemoRT). Of the 628 patients, 373 (59%) were men, 255 (41%) were women, and the mean age was 56.7 years.
RESULTS: Patients in the TNT cohort received greater percentages of the planned oxaliplatin and fluorouracil prescribed dose than those in the chemoRT with plannned adjuvant chemotherapy cohort. The complete response (CR) rate, including both pathologic CR (pCR) in those who underwent surgery and sustained clinical CR (cCR) for at least 12 months posttreatment in those who did not undergo surgery, was 36% in the TNT cohort compared with 21% in the chemoRT with planned adjuvant chemotherapy cohort. 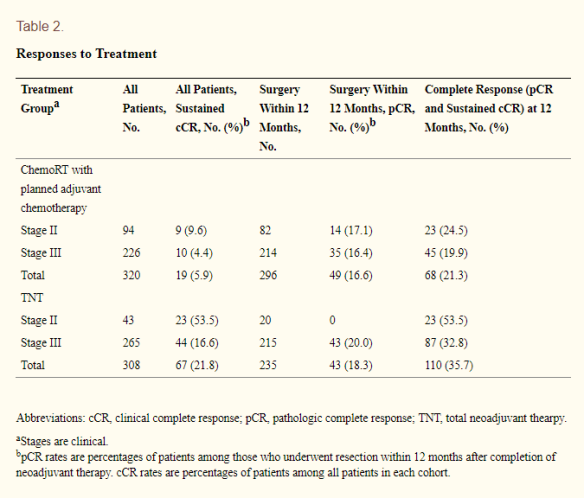 (Cerek et al, 2018)
(Cerek et al, 2018)
CONCLUSIONS: Total neoadjuvant therapy was associated with improved delivery of systemic therapy and increased response to treatment, and it provides a promising platform for nonoperative watch-and-wait protocols. Long-term follow-up is necessary to determine if early systemic chemotherapy improves overall outcome
The authors conclude their findings provide additional support for the National Comprehensive Cancer Network (NCCN) guidelines that categorize TNT as a viable treatment strategy for rectal cancer.


