One discussion this week included the TRICC trial.
Reference: Herbert PC, et al…the Transfusion Requirements in Critical Care Investigators for the Canadian Critical Care Trials Group. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. NEJM. 1999 Feb 11;340(6):409-417.
Summary: The aim of the study was to determine whether a restrictive strategy of red-cell transfusion and a liberal strategy produced equivalent results in critically ill patients, we compared the rates of death from all causes at 30 days and the severity of organ dysfunction.
Methods: Between 1994 and 1997, the trial enrolled 838 critically ill patients with euvolemia after initial treatment who had hemoglobin concentrations of less than 9.0 g per deciliter within 72 hours after admission to the intensive care unit and randomly assigned 418 patients to a restrictive strategy of transfusion, in which red cells were transfused if the hemoglobin concentration dropped below 7.0 g per deciliter and hemoglobin concentrations were maintained at 7.0 to 9.0 g per deciliter, and 420 patients to a liberal strategy, in which transfusions were given when the hemoglobin concentration fell below 10.0 g per deciliter and hemoglobin concentrations were maintained at 10.0 to 12.0 g per deciliter.
Results: The use of a threshold for red-cell transfusion as low as 7.0 g of hemoglobin per deciliter, combined with maintenance of hemoglobin concentrations in the range of 7.0 to 9.0 g per deciliter, was at least as effective as and possibly superior to a liberal transfusion strategy (threshold, 10.0 g per deciliter; maintenance range, 10.0 to 12.0) in critically ill patients with normovolemia. There was a trend toward decreased 30-day mortality among patients who were treated according to the restrictive transfusion strategy. The significant differences in mortality rates during hospitalization, rates of cardiac complications, and rates of organ dysfunction all favored the restrictive strategy.
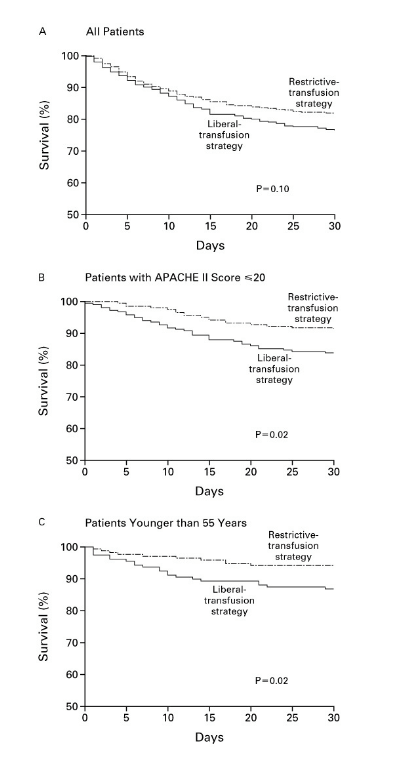
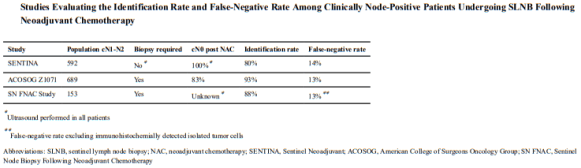
Overall, 30-day mortality was similar in the two groups (18.7 percent vs. 23.3 percent, P= 0.11). However, the rates were significantly lower with the restrictive transfusion strategy among patients who were less acutely ill — those with an Acute Physiology and Chronic Health Evaluation II score of < or =20 (8.7 percent in the restrictive-strategy group and 16.1 percent in the liberal-strategy group; P=0.03) — and among patients who were less than 55 years of age (5.7 percent and 13.0 percent, respectively; P=0.02), but not among patients with clinically significant cardiac disease (20.5 percent and 22.9 percent, respectively; P=0.69). The mortality rate during hospitalization was significantly lower in the restrictive-strategy group (22.3 percent vs. 28.1 percent, P=0.05).
Conclusion: On the basis of the trial’s results, the authors recommend that critically ill patients receive red-cell transfusions when their hemoglobin concentrations fall below 7.0 g per deciliter and that hemoglobin concentrations should be maintained between 7.0 and 9.0 g per deciliter. The diversity of the patients enrolled in this trial and the consistency of the results suggest that these conclusions may be generalized to most critically ill patients, with the possible exception of patients with active coronary ischemic syndromes.