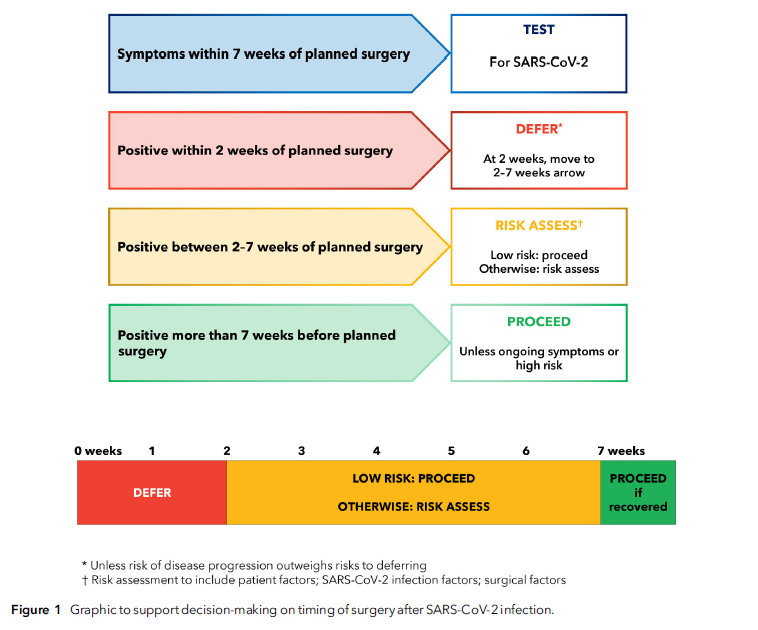
“Patients who develop symptoms of SARS-CoV-2 infection within 7 weeks of planned surgery, including on the day of surgery, should be screened for SARS-CoV-2. Elective surgery should not usually be undertaken within 2 weeks of diagnosis of SARS-CoV-2 infection. For patients who have recovered from SARS-CoV-2 infection and who are low risk or having low-risk surgery, most elective surgery can proceed 2 weeks following a SARS-CoV-2 positive test. For patients who are not low risk or having anything other than low-risk surgery between 2 and 7 weeks following infection, an individual risk assessment must be performed. This should consider: patient factors (age; comorbid and functional status); infection factors (severity; ongoing symptoms; vaccination); and surgical factors (clinical priority; risk of disease progression; grade of surgery).”