“Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are recommended for patients with type 2 diabetes to control glycemia and reduce cardiovascular risk, and for patients with obesity to reduce weight. Given the wide-spread use of these drugs, potential safety concerns deserve attention.
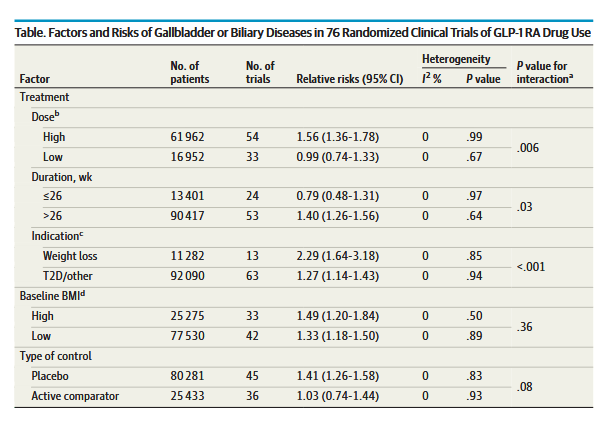
Several randomized clinical trials (RCTs) have shown a higher rate of gallbladder disorders in patients who were randomized to GLP-1 RAs vs a placebo. However, whether
increased risk of gallbladder-related events is a class effect of GLP-1 RAs has not been established, and prescribing information for all GLP-1 RA medications does not provide a warning regarding increased risk of gallbladder disorders. In addition to gallbladder-related events, a post hoc analysis of the LEADER trial 8 found significantly increased risks of acute biliary obstruction in patients randomized to liraglutide compared with placebo. Because
GLP-1 RAs are generally prescribed at higher doses for weight loss rather than for control of type 2 diabetes, there may be differential effects on risk for gallbladder or biliary diseases depending on dose.”