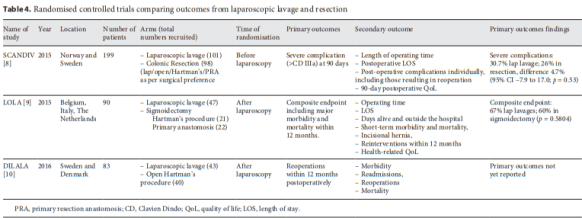
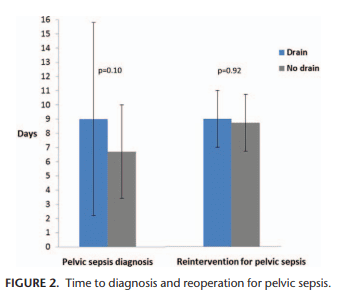
One discussion this week involved the surgical management of Crohn’s Disease.
Reference: Strong S, et al. Clinical practice guideline for the surgical management of Crohn’s Disease. Diseases of the Colon and Rectum. 2015 Nov;58(11):1021-1036. doi:10.1097/DCR.0000000000000450
Summary: The authors state “these guidelines should not be deemed inclusive of all proper methods of care or exclusive of methods of care reasonably directed to obtaining the same results. The ultimate judgment regarding the propriety of any specific procedure must be made by the physician in light of all the circumstances presented by the individual patient” (p.1021).
OPERATIVE INDICATIONS
Failed Medical Therapy
- Patients who demonstrate an inadequate response to, develop complications from, or are noncompliant with medical therapy should be considered for surgery. Grade of Recommendation: Strong based on low-or very low-quality evidence, 1C.
- Patients receiving therapy with anti-TNFs, high-dose glucocorticoids and/or cyclosporine may warrant staged procedures because of concerns about postoperative complications; however, decisions should be individualized based on the patient’s risk stratification, overall clinical status, and surgeon judgment. Grady of Recommendation: Weak based on low- or very low-quality evidence, 2C.
Inflammation
- Patient with acute colitis who have symptoms or signs of impending or actual perforation should typically undergo surgery. Grade of Recommendation: Strong based on low- or very low-quality evidence, 1C.
Stricture
- Endoscopic dilation may be considered for patients with symptomatic small-bowel or anastomotic strictures that are not amenable to medical therapy. Grade of Recommendation: Strong based on low- or very low-quality evidence, 1C.
- Surgery is indicated for patients with symptomatic small-bowel or anastomotic strictures that are not amenable to medical therapy and/or dilation. Grade of Recommendation: Strong based on low- or very low-quality evidence, 1C.
- Patients with strictures of the colon that cannot be adequately surveyed endoscopically should be considered for resection. Grade of Recommendation: Strong based on low- or very-low quality evidence, 1C.
Penetrating Disease
- Patients with a free perforation should undergo surgery. Grade of Recommendation: Strong based on moderate-quality evidence, 1B.
- Patients with enteroparietal, interloop, intramesenteric, or retroperitoneal abscesses may be managed by antibiotics with or without percutaneous drainage. Surgical drainage with or without resection should be considered when this is not successful. Grade of Recommendation: Weak based on moderate-quality evidence, 2B.
- Patients with enteric fistulas and symptoms or signs of localized or systemic sepsis that persist despite appropriate medical therapy should be considered for surgery. Grade of Recommendation: Strong based on low- or very low-quality evidence, 1C.
Hemorrhage
- Stable patients with significant GI heomrrhage may be evaluated and treated by endoscopic and/or interventional radiological techniques. Unstable patients should typically undergo operative exploration. Grade of Recommendation: STrong based on low- or very low-quality evidence, 1C.
Growth Retardation
- Prepubertal patients with significant growth retardation despite appropriate medical therapy should be considered for surgery. Grade of Recommendation: STrong based on moderate-quality evidence, 1B.
Neoplasia
- Patients with long-standing Crohn’s disease of the ileocolic region or colon should have endoscopic surveillance of the large bowel. Grade of Recommendation: Strong based on moderate-quality evidence, 1B.
- Total proctocolectomy should be considered for patients with carcinoma, a nonadenoma-like dysplasi-associated lesion or mass (DALM), high-grade dysplasia, or multifocal low-grade dysplasia of the colon or rectum. Grade of Recommendation: Strong based on moderate-quality evidence, 1B.
- Suspicious lesions (mass, ulcer) identified in patients with Crohn’s should typically be biopsied, especially when considering a small-bowel strictureplasty. Grade of Recommendation: Strong based on low- or very low-quality evidence.
For complete guidelines (site-specific operations, technical considerations), methodologies, and definition of GRADE system-grading recommendations, see full text article.